Working from the electrochemical equivalents chart in the mfg it takes 143 asf for 60 minutes to deposit001 of zinc at 100 efficiency. The zinc sulfate and zinc electrodes were purchased on ebay for less than 20.

Zinc Electroplating Products Finishing
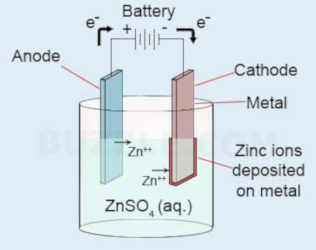
Zinc plating solution formula. The sugar is not part of the balanced formula for the chemical reaction. The reaction at the anode is that for each 2 electrons that your battery removes one atom of zinc goes into solution according to the reaction zn0 2e zn. Zinc electroplating steps although the process may vary depending on the requirements substrate cost and the type of finish desired at a commercial level it usually involves the following major steps. After a half century of use they were in poor condition and required replating. So at 10 asf it will take about 34 minutes. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating.
For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. Place the anodes into the solution. Sand particles breach exposed surfaces causing erosion and surface cavities. Rather it blocks continued plating and crystal growth inducing the formation of new crystals. A recent price check on ebay found 2 pounds of zinc sulfate for 899 and 4 zinc anodes for 876 total cost with delivery while not particularly dangerous zinc sulfate plating solution and cleaning materials are irritants. Your efficiency should be 75 maybe a bit higher so 14375 19 asf for 60 minutes to deposit001 or 57 asf for 60 minutes to deposit 00003.
Sending them out for replating wasnt practical so i set up my own zinc plating system. This section describes my experiments in creating that system. Vinegar is a weak acid and dissolves the zinc. The resulting ph is about 3 as determined by litmus paper. Mix together 300 grams of epsom salts 100 grams of zinc sulfate 200ml of white vinegar and about 13 bottle of corn syrup into 45 liters of tap water and stir with a paint stick until everything is well dissolved. Zinc nickel plating therefore has become the corrosion resistant solution for automotive components to protect against these environments.
Zinc nickel has received increasing commercial acceptance over the past 20 years. Many of the parts removed from the triumph were originally zinc plated or galvanized. This generates zinc ions in the vinegar. Zinc plating is done in a variety of aqueous solutions like alkaline cyanide alkaline non cyanide or acid chloride salt solutions.